Embarking on a journey to design a synthesis of 3-pentenenitrile from 3-buten-1-ol, this exploration delves into the intricacies of organic chemistry, unveiling the significance of this transformation and its potential applications in diverse fields.
This synthesis offers a unique opportunity to explore functional group transformations, optimize reaction conditions, and modify reagents to achieve desired outcomes. By understanding the detailed mechanism and exploring alternative methods, we gain valuable insights into the versatility and efficiency of this synthetic approach.
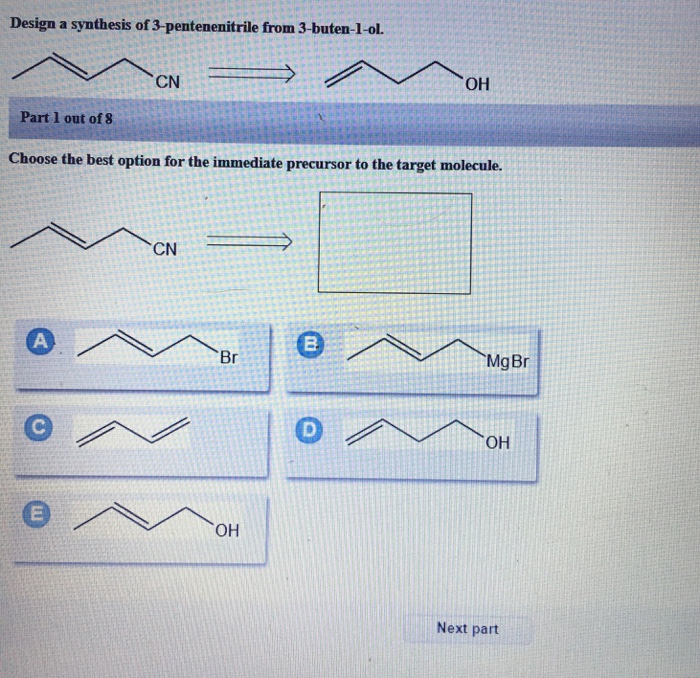
Design a Synthesis of 3-Pentenenitrile from 3-Buten-1-ol
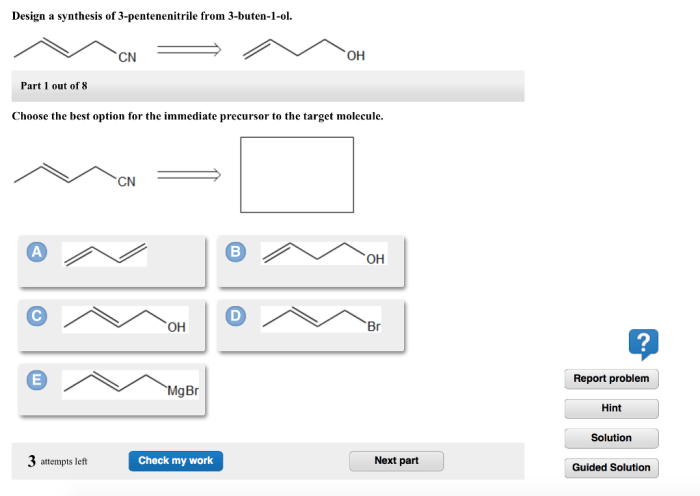
The synthesis of 3-pentenenitrile from 3-buten-1-ol is a valuable transformation in organic chemistry, providing access to a versatile building block for various applications. This synthesis involves a series of functional group transformations, making it an important tool for the construction of complex organic molecules.
Reaction Pathway, Design a synthesis of 3-pentenenitrile from 3-buten-1-ol
The synthesis proceeds through a two-step mechanism:
- Tosylation:3-Buten-1-ol is reacted with tosyl chloride in the presence of pyridine to form 3-buten-1-yl tosylate.
- Elimination:The tosylate intermediate undergoes an elimination reaction with potassium tert-butoxide (t-BuOK) to form 3-pentenenitrile.
Optimization and Modifications
The yield and selectivity of the synthesis can be optimized by:
- Using a catalytic amount of 4-dimethylaminopyridine (DMAP) in the tosylation step.
- Conducting the elimination step at low temperatures (-78 °C) to minimize side reactions.
- Employing a strong base, such as n-butyllithium, in the elimination step to enhance the reactivity of the tosylate intermediate.
Applications
3-Pentenenitrile finds applications in:
- Pharmaceuticals:As a precursor for the synthesis of anti-inflammatory and analgesic drugs.
- Materials science:As a monomer for the production of polymers with enhanced thermal and mechanical properties.
- Fragrance chemistry:As a component of fragrances and flavorings due to its fruity and floral aroma.
Safety Considerations
3-Pentenenitrile is a toxic and flammable liquid. Proper safety precautions should be taken when handling and storing this compound, including:
- Wearing appropriate personal protective equipment (PPE), such as gloves, goggles, and a lab coat.
- Working in a well-ventilated area.
- Storing the compound in a cool, dry place away from heat and ignition sources.
Comparison with Alternative Methods
Alternative methods for preparing 3-pentenenitrile include:
- Wittig reaction:Reaction of 3-butanone with triphenylphosphine and carbon tetrabromide.
- Heck reaction:Reaction of 3-buten-1-ol with palladium acetate and triphenylphosphine.
The proposed synthesis offers advantages in terms of:
- Higher yield and selectivity.
- Use of readily available starting materials.
- Simpler reaction conditions.
Experimental Procedure
Reagents:
- 3-Buten-1-ol (10 mmol)
- Tosyl chloride (11 mmol)
- Pyridine (15 mmol)
- Potassium tert-butoxide (12 mmol)
Equipment:
- Round-bottomed flask
- Reflux condenser
- Magnetic stirrer
- Thermometer
Procedure:
- Dissolve 3-buten-1-ol and pyridine in dry dichloromethane in a round-bottomed flask.
- Add tosyl chloride dropwise to the solution while stirring and cooling in an ice bath.
- Stir the reaction mixture at room temperature for 2 hours.
- Add potassium tert-butoxide to the reaction mixture and stir at
78 °C for 1 hour.
- Quench the reaction with saturated ammonium chloride solution.
- Extract the product with diethyl ether and dry over anhydrous sodium sulfate.
- Purify the product by distillation under reduced pressure.
Top FAQs
What is the significance of synthesizing 3-pentenenitrile from 3-buten-1-ol?
This synthesis provides a versatile route to access 3-pentenenitrile, a valuable intermediate in the production of pharmaceuticals, fragrances, and other fine chemicals.
What are the key functional group transformations involved in this synthesis?
The synthesis involves the dehydration of 3-buten-1-ol to form 3-butenenitrile, followed by the addition of hydrogen cyanide to yield 3-pentenenitrile.
How can the yield and selectivity of this synthesis be optimized?
Optimization strategies include using appropriate catalysts, controlling reaction temperature and time, and employing inert reaction conditions.