The Bohr model and electromagnetic spectrum practice delve into the enigmatic realms of atomic structure and the boundless expanse of cosmic energy, unraveling the secrets of the physical world. From the quantization of energy levels within atoms to the vibrant hues of the celestial rainbow, this exploration unveils the intricate tapestry that weaves together the fundamental building blocks of matter and the vastness of the universe.
Through a comprehensive examination of the Bohr model’s revolutionary insights and the multifaceted nature of the electromagnetic spectrum, we embark on a journey that illuminates the very essence of our physical reality.
Bohr Model
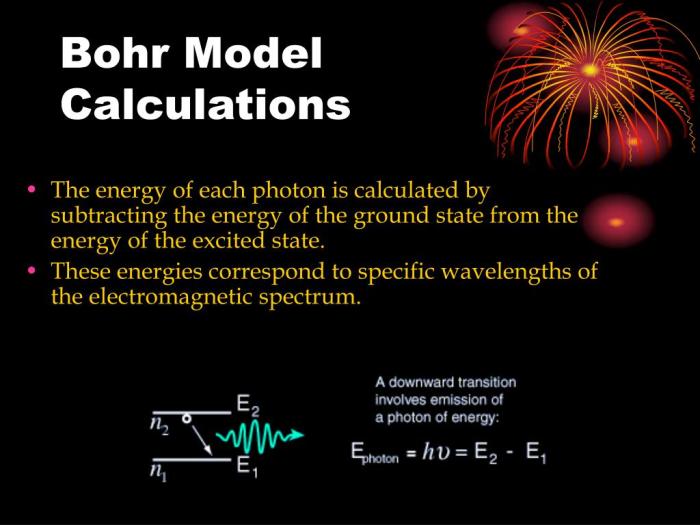
The Bohr model of the atom, proposed by Niels Bohr in 1913, revolutionized our understanding of atomic structure. It introduced the concept of energy levels and explained the emission and absorption of light by atoms.
According to the Bohr model, electrons orbit the nucleus in discrete energy levels. Each energy level has a specific radius and energy. Electrons can transition between energy levels by absorbing or emitting photons of light with energy equal to the difference in energy between the two levels.
Limitations of the Bohr Model
- The Bohr model does not explain the fine structure of spectral lines, which is observed in experiments.
- It does not account for the wave-particle duality of electrons.
- It does not explain the chemical bonding between atoms.
Electromagnetic Spectrum: Bohr Model And Electromagnetic Spectrum Practice
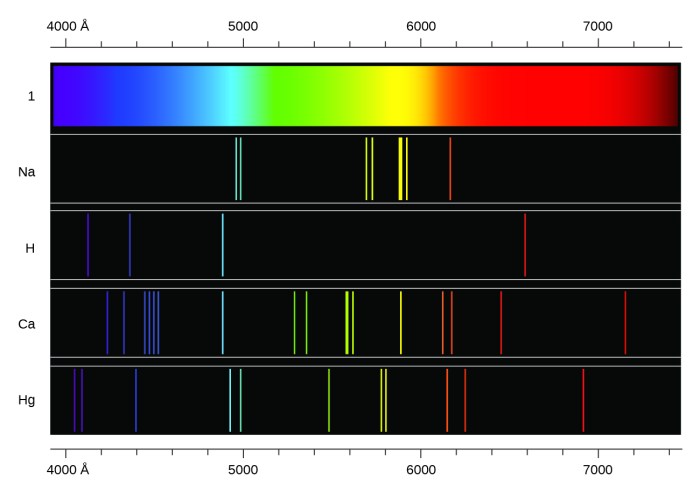
The electromagnetic spectrum is the range of all possible frequencies of electromagnetic radiation. It includes radio waves, microwaves, infrared radiation, visible light, ultraviolet radiation, X-rays, and gamma rays.
Different Regions of the Electromagnetic Spectrum
- Radio waves: The longest wavelength region, used for communication, navigation, and remote sensing.
- Microwaves: Used for cooking, heating, and radar systems.
- Infrared radiation: Used for heat detection, thermal imaging, and spectroscopy.
- Visible light: The only region visible to the human eye, used for vision and communication.
- Ultraviolet radiation: Used for sterilization, tanning, and phototherapy.
- X-rays: Used for medical imaging, security screening, and crystallography.
- Gamma rays: The shortest wavelength region, used for medical imaging, cancer therapy, and nuclear physics.
Uses of the Electromagnetic Spectrum
- Communication: Radio waves, microwaves, and visible light are used for wireless communication.
- Medical imaging: X-rays and gamma rays are used for medical diagnostics and therapy.
- Remote sensing: Microwaves and infrared radiation are used for weather forecasting, environmental monitoring, and land use planning.
Practice Problems
Bohr Model, Bohr model and electromagnetic spectrum practice
Calculate the energy of a photon emitted when an electron transitions from the n = 3 energy level to the n = 1 energy level in a hydrogen atom.
Solution:
The energy of a photon is given by the equation E = hf, where h is Planck’s constant and f is the frequency of the photon. The frequency of the photon emitted is given by the difference in energy between the two energy levels:
f = (E 3– E 1) / h
The energy of the n = 3 energy level is given by:
E 3= -13.6 eV / 3 2= -1.51 eV
The energy of the n = 1 energy level is given by:
E 1= -13.6 eV / 1 2= -13.6 eV
Therefore, the energy of the photon emitted is:
E = hf = (E 3– E 1) / h = (-1.51 eV – (-13.6 eV)) / h = 12.09 eV
Electromagnetic Spectrum
What type of electromagnetic radiation has a wavelength of 10 -10m?
Solution:
The wavelength of 10 -10m corresponds to the X-ray region of the electromagnetic spectrum.
Applications
Bohr Model, Bohr model and electromagnetic spectrum practice
- Understanding atomic structure and the emission and absorption of light.
- Developing lasers and other light-based technologies.
- Explaining the chemical bonding between atoms.
Electromagnetic Spectrum
- Communication and data transmission.
- Medical imaging and diagnosis.
- Remote sensing and environmental monitoring.
- Industrial and scientific research.
Top FAQs
What is the significance of the Bohr model?
The Bohr model revolutionized our understanding of atomic structure by introducing the concept of quantized energy levels, providing a foundation for modern quantum mechanics.
How is the electromagnetic spectrum classified?
The electromagnetic spectrum is typically divided into seven regions: radio waves, microwaves, infrared radiation, visible light, ultraviolet radiation, X-rays, and gamma rays, each with distinct properties and applications.
What are some practical applications of the electromagnetic spectrum?
The electromagnetic spectrum finds widespread use in various fields, including telecommunications, medical imaging, remote sensing, and energy production.